Current Issue
Inferior Alveolar Nerve Regeneration Techniques: A Literature Review
Adel Bouguezzi*, Afef Slim, Chaima Khalifa, Chokri Abdellatif, Hajer Hentati, Jamil Selmi
University Dental Clinic, Medicine and Oral Surgery Department, Oral Health and Orofacial Rehabilitation Laboratory Research (LR12ES11), University of Monastir, Tunisia
*Corresponding author: Adel Bouguezzi, University Dental Clinic, Medicine and Oral Surgery Department, Oral Health and Orofacial Rehabilitation Laboratory Research (LR12ES11), University of Monastir, Tunisia, E-mail: [email protected]
Received Date: January 03, 2025
Publication Date: January 27, 2025
Citation: Bouguezzi A, et al. (2025). Inferior Alveolar Nerve Regeneration Techniques: A Literature Review. Dental. 7(1):18.
Copyright: Bouguezzi A, et al. © (2025).
ABSTRACT
Inferior alveolar nerve (IAN) injury is a common complication in oral and maxillofacial surgery, often resulting in sensory deficits such as paresthesia, hypoesthesia, and dysesthesia. The management and regeneration of the IAN following trauma or surgical injury present significant challenges. Advances in nerve regeneration techniques, including autologous grafting, alloplastic conduits, platelet-rich plasma (PRP), stem cell therapies, and pharmacological agents, have shown promising results in clinical and preclinical studies. This comprehensive review explores the current literature on IAN regeneration, providing insights into the mechanisms, techniques, and future directions for improving patient outcomes.
Keywords: Inferior Alveolar Nerve, Nerve Regeneration, Autologous Graft, Nerve Conduits, Stem Cell Therapy, Platelet-Rich Plasma, Maxillofacial Surgery
INTRODUCTION
The inferior alveolar nerve (IAN) plays a critical role in providing sensory innervation to the lower lip, chin, and teeth. Its injury, often associated with third molar extractions, dental implant placements, and mandibular fractures, can result in debilitating sensory disturbances that significantly impact patients' quality of life. The complexity of nerve repair arises from the limited regenerative capacity of peripheral nerves and the intricate anatomical course of the IAN [1].
Over the past few decades, considerable research has been devoted to developing techniques to enhance nerve regeneration, minimize sensory deficits, and restore nerve function. This review aims to synthesize current advancements in IAN regeneration, examining their efficacy, mechanisms, and potential for clinical translation.
ANATOMY AND PATHOPHYSIOLOGY OF IAN INJURY
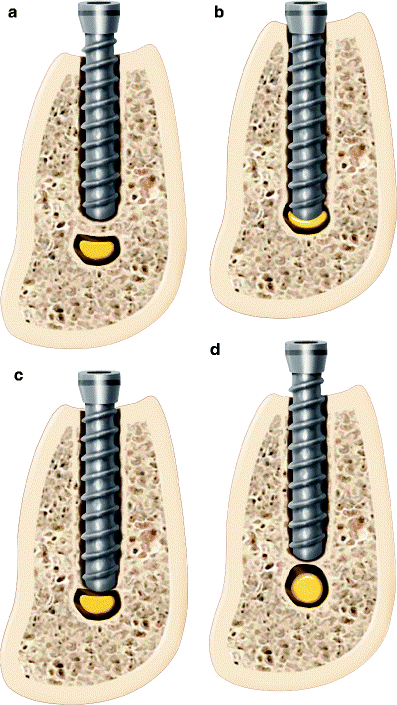
The IAN is a branch of the mandibular division of the trigeminal nerve (cranial nerve V). It travels through the mandibular canal and exits via the mental foramen to supply the chin and lower lip. Due to its anatomical proximity to the roots of the mandibular third molar and the inferior border of the mandible, the IAN is particularly susceptible to injury during oral surgical procedures [2] (Figure 1).
IAN injuries are classified into three types: neuropraxia (temporary conduction block), axonotmesis (axonal disruption with intact nerve sheath), and neurotmesis (complete nerve severance). While neuropraxia may resolve spontaneously, axonotmesis and neurotmesis often require surgical intervention to restore function [3].
Figure 1. (a) Collapse of the superior aspect of the IAC due to implant placement beyond the planned osteotomy causing injury to the nerve (compartment syndrome). (b) Direct injury to the IAN by implant contact. (c) Direct injury to the cortical rim of the IAC with deformation of the neurovascular bundle. (d) Remodeling of the IAC cortical rim causing narrowing of canal.
NERVE REGENERATION MECHANISMS
Nerve regeneration involves a complex interplay of cellular and molecular events, including Schwann cell proliferation, axonal sprouting, and remyelination. Schwann cells play a pivotal role by creating a permissive environment for axonal regrowth and secreting neurotrophic factors such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) [4].
Following nerve injury, Schwann cells undergo phenotypic changes, dedifferentiating into a repair-promoting state. These cells align to form bands of Büngner, guiding regenerating axons toward the target tissue. Additionally, they secrete extracellular matrix proteins such as laminin and fibronectin, which facilitate axonal attachment and elongation. The inflammatory response following nerve injury is essential for regeneration but must be tightly regulated. Macrophages play a dual role by clearing myelin debris through phagocytosis while releasing cytokines and chemokines that promote Schwann cell migration. Excessive inflammation, however, can lead to fibrosis, hindering axonal growth [5].
Neurotrophic factors, including NGF, BDNF, and glial cell line-derived neurotrophic factor (GDNF), stimulate neuronal survival and axonal elongation by activating intracellular signaling cascades such as the PI3K/Akt and MAPK/ERK pathways. This signaling promotes cytoskeletal rearrangement and growth cone advancement. Remyelination of regenerating axons is critical for restoring nerve conduction velocity [6]. Despite the intrinsic regenerative capacity of peripheral nerves, large nerve gaps and misaligned fibers remain significant challenges. This necessitates adjunctive strategies such as nerve grafts, conduits, and biomaterials to bridge the defect and optimize the regenerative environment [7].
TECHNIQUES FOR IAN REGENERATION
Autologous Nerve Grafting
Autologous nerve grafting is the gold standard for managing nerve defects larger than 5 mm. This technique involves harvesting a segment of nerve tissue from the patient, commonly from donor sites such as the sural, great auricular, or mental nerves. The harvested nerve is sutured into the defect site to serve as a scaffold for axonal regrowth, allowing the regenerating nerve fibers to traverse the gap [8].
Advantages
Autologous grafting boasts high biocompatibility, reducing the risk of immune rejection and enhancing integration with surrounding tissues. The graft provides not only structural support but also Schwann cells that promote axonal regeneration. This technique consistently yields high success rates for large nerve defects, with favorable long-term sensory and functional outcomes.
Disadvantages
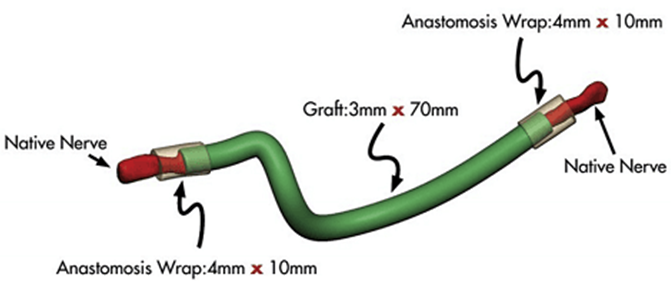
However, autologous nerve grafting is associated with donor site morbidity, often resulting in sensory deficits, scarring, or pain at the harvest site. The length of the graft available limits its use for extensive nerve injuries, and there is a risk of neuroma formation or incomplete regeneration if alignment is not meticulously maintained [8](Figure 2)
Figure 2. llustration of using an avance nerve graft.
Alloplastic Nerve Conduits
Alloplastic nerve conduits present a less invasive alternative to autologous grafts. These conduits, fabricated from biocompatible materials like polyglycolic acid (PGA) or collagen, provide a tubular scaffold that bridges nerve gaps and guides axonal regrowth [9].
Advantages
The primary benefits of nerve conduits include reduced surgical morbidity and avoidance of donor site complications. These conduits minimize surgical time and are well-suited for smaller nerve gaps (typically less than 10 mm).
Disadvantages
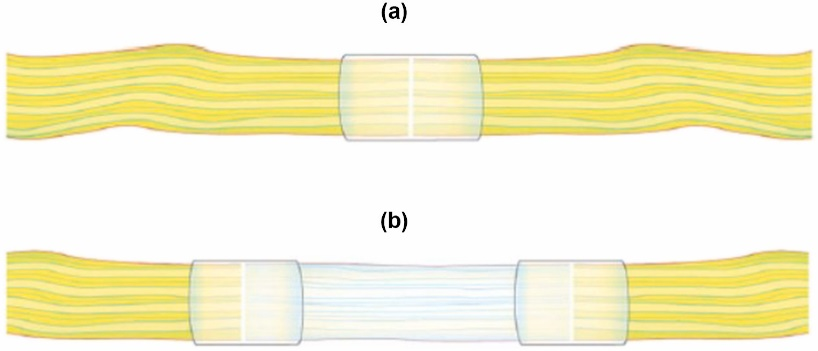
However, nerve conduits are limited in application to smaller defects. Larger gaps may result in inconsistent long-term sensory recovery. Additionally, the lack of Schwann cells within the conduit may slow the regeneration process, reducing the efficacy of this method compared to autologous grafting [9](Figure 3).
Figure 3. A.Diagram of a connector-assisted direct nerve repair. B. Diagram of a connector-assisted allograft repair [9].
Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF)
PRP and PRF have gained popularity as adjunctive therapies for nerve regeneration due to their high concentration of growth factors, which accelerate healing and promote tissue regeneration. These autologous blood products are prepared by centrifuging the patient's blood to separate and concentrate platelets and fibrin. Once applied to the nerve injury site, PRP and PRF stimulate Schwann cell proliferation, enhance angiogenesis, and create a bioactive scaffold that facilitates axonal growth [10].
Advantages
PRP and PRF are easy to obtain and apply, with minimal risk of immune rejection. They enhance the natural healing process, reduce inflammation, and promote faster nerve regeneration. Clinical studies have demonstrated improved sensory recovery and reduced postoperative pain in patients treated with PRP/PRF following nerve injury. Additionally, these techniques are cost-effective and can be combined with other nerve repair methods.
Disadvantages
Despite their benefits, PRP and PRF therapies require multiple applications to achieve optimal results. The variability in platelet concentration, depending on the preparation technique, may lead to inconsistent outcomes. Furthermore, the regenerative effects are generally limited to smaller nerve gaps, and the lack of structural support limits their efficacy for larger nerve defects [10].
Stem Cell Therapy
Stem cell therapy represents a cutting-edge approach to nerve regeneration, leveraging the regenerative potential of mesenchymal stem cells (MSCs) derived from bone marrow, adipose tissue, or dental pulp. These cells can differentiate into Schwann-like cells, secrete neurotrophic factors, and modulate the inflammatory environment to promote axonal regrowth [11].
Advantages
Stem cell therapy has shown promising results in preclinical studies, demonstrating enhanced nerve regeneration, reduced scarring, and functional recovery. The ability to harvest MSCs from various sources provides flexibility and reduces the need for invasive procedures. Additionally, stem cells can be combined with nerve conduits or PRP to create a synergistic effect, enhancing overall outcomes.
Disadvantages
Despite its potential, stem cell therapy is still in the experimental stages and has yet to become a standard clinical practice. Challenges include high costs, ethical concerns related to stem cell sourcing, and the risk of uncontrolled cell proliferation. The complexity of stem cell culture and delivery methods also limits widespread application [11].
Pharmacological Agents and Neurotrophic Factors
Pharmacological agents and neurotrophic factors such as NGF, BDNF, and glial cell line-derived neurotrophic factor (GDNF) play essential roles in promoting axonal growth and preventing apoptosis. Tacrolimus, an immunosuppressant, has demonstrated neuroregenerative properties by enhancing Schwann cell proliferation and increasing the expression of neurotrophic factors [12].
Advantages
These agents provide a non-invasive approach to nerve regeneration, enhancing the body's natural repair mechanisms. Pharmacological therapies can be administered systemically or locally, making them versatile and accessible. Additionally, combining neurotrophic factors with other regenerative techniques, such as grafting or conduits, further improves outcomes.
Disadvantages
Systemic administration of pharmacological agents may lead to side effects, limiting their long-term use. The effectiveness of neurotrophic factors is dose-dependent, and maintaining consistent therapeutic levels can be challenging. Furthermore, these agents alone are insufficient for repairing large nerve defects, necessitating combination therapies [12].
FUTURE DIRECTIONS
The future of IAN regeneration lies in developing hybrid approaches that integrate multiple techniques to enhance nerve repair. Combining stem cell therapy with PRF or conduits has shown synergistic effects in preclinical models, accelerating axonal growth and reducing nerve gaps [13]. Advances in 3D-printed scaffolds and bioengineered nerve conduits are poised to revolutionize nerve regeneration by providing customized solutions for complex nerve injuries.
Gene therapy, aimed at enhancing the expression of neurotrophic factors and modulating inflammatory responses, holds significant potential for improving nerve regeneration outcomes. Research is also focusing on electrical stimulation and biomaterials that mimic the natural extracellular matrix, further enhancing axonal guidance and remyelination [13].
Clinical trials exploring the long-term efficacy of these emerging therapies are crucial to translating preclinical findings into standard practice. The integration of advanced imaging technologies, such as intraoperative nerve monitoring and high-resolution MRI, will further refine surgical techniques and reduce the risk of IAN injury during oral and maxillofacial procedures [14].
CONCLUSION
Inferior alveolar nerve regeneration remains a challenging aspect of oral and maxillofacial surgery. While autologous nerve grafting continues to be the gold standard for large nerve defects, emerging techniques such as PRF, stem cell therapy, and pharmacological agents provide promising adjuncts to enhance nerve healing. Future advancements in biomaterials, gene therapy, and tissue engineering are expected to revolutionize the field, ultimately improving patient outcomes and quality of life.
ACKNOWLEDGMENTS
None.
CONFLICTS OF INTEREST
The authors declare that no conflict of interest.
REFERENCES
- Nishiyama A, Odaka K, Koyachi M, Sugahara K, Katakura A. (2022). Alternative technique to repair damaged inferior alveolar nerve using data fusion from computed tomographic and magnetic resonance imaging. Br J Oral Maxillofac Surg. 60(2):207-208.
- Navia A, Tapia S, Rojas MF, Rojas F, Vargas A, Guerra C, et al. (2024). Repair of Inferior Alveolar Nerve in Orthognathic Surgery Simulator (RIANOS): A Novel, Open-Source, Combined 3D Printed, and Ex-Vivo Chicken Sciatic Nerve Training Model. Craniomaxillofac Trauma Reconstr. 9433875241236322.
- Siemionow MZ. (2010). Plastic and Reconstructive Surgery. New York, NY, Springer.
- Strauss ER, Ziccardi VB, Janal MN. (2006). Outcome assessment of inferior alveolar nerve microsurgery: a retrospective review. J Oral Maxillofac Surg. 64(12):1767-1770.
- Meyer RA, Bagheri SC. (2011). Clinical evaluation of peripheral trigeminal nerve injuries. Atlas Oral Maxillofac Surg Clin North Am. 19(1):15-33.
- Callahan N, Houle A, Miloro M. (2022). DANG (Depth-Adjusted Nerve Guide) - A Technical Note. J Oral Maxillofac Surg. 80(1):197-199.
- Peleg O, Mahmoud R, Shuster A, Arbel S, Manor Y, Ianculovici C, et al. (2021). Orthognathic surgery complications: The 10-year experience of a single center. J Craniomaxillofac Surg. 49(10):891-897.
- Miloro M. (1995). Surgical access for inferior alveolar nerve repair. J Oral Maxillofac Surg. 53(10):1224-1225.
- Lavoie S, Dolivo J. (2019). Platelet-Rich Plasma and Platelet-Rich Fibrin in Peripheral Nerve Regeneration: Mechanisms and Clinical Applications. Journal of Tissue Engineering and Regenerative Medicine. 13(12) :1912-1924.
- Kraeima J, Dorgelo B, Gulbitti HA, Steenbakkers RJHM, Schepman KP, Roodenburg JLN, et al. (2018). Multi-modality 3D mandibular resection planning in head and neck cancer using CT and MRI data fusion: A clinical series. Oral Oncol. 81:22-28.
- Miloro M, Halkias LE, Slone HW, Chakeres DW. (1997). Assessment of the lingual nerve in the third molar region using magnetic resonance imaging. J Oral Maxillofac Surg. 55(2):134-137.
- Yampolsky A, Ziccardi V, Chuang SK. (2017). Efficacy of Acellular Nerve Allografts in Trigeminal Nerve Reconstruction. J Oral Maxillofac Surg. 75(10):2230-2234.
- Safa B, Shores JT, Ingari JV, Weber RV, Cho M, Zoldos J, et al. (2019). Recovery of Motor Function after Mixed and Motor Nerve Repair with Processed Nerve Allograft. Plast Reconstr Surg Glob Open. 7(3):e2163.
- Leite GB, Sartoretto SC, Lima FCA, Calasans-Maia M, Louro RS. (2017). The Pull-Through Technique: A Viable Option for Preserving the Inferior Alveolar Nerve during Surgical Resection. Craniomaxillofac Trauma Reconstr. 10(4):329-331.
 Abstract
Abstract  PDF
PDF